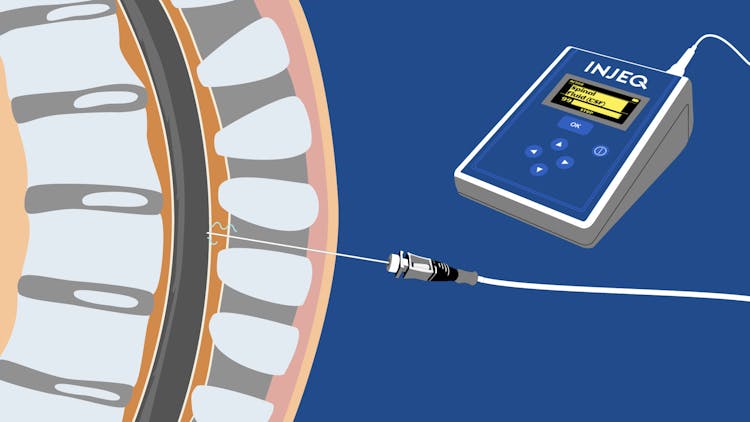
"Injeq needle has generated enthusiastic feedback from doctors all over the world. The sales of the present Injeq IQ-Tip® needle system has been challenging due to its price point- despite it's clear clinically proven advantages. Injeq's management has recognised the need to push the price point closer to that of regular needles to lower the barrier to enter new markets, scale the sales to a global level and reach a material market position as soon as possible. Injeq has started developing the Injeq 2.0 analyzer which has a substantially lower price point and allows Injeq to penetrate the market from a completely different angle. Injeq 2.0 analyzer is currently in laboratory tests at Injeq and it is expected to be on the market in 2025. At the same time Injeq is expecting to obtain FDA approval in 2026 which opens a completely new market for Injeq where the need for the highest quality products with the lowest risk for patients is resulting to a winning formula.
Injeq is now raising €2-3M to finalise the Injeq 2.0 analyzer, clinical trials, FDA approval and global expansion. Injeq is convinced that the Injeq 2.0 analyzer will allow Injeq to expand rapidly on a global level driven by the lower price point and superiority of the needle compared to regular needles. The €2M minimum funding will also allow Injeq to fully convert the convertible bond which Injeq issued in 2022 after Injeq's planned IPO was discontinued due to Russia's invasion of Ukraine. In addition to enable Injeq to convert the convertible bond the new funding is expected to give Injeq a runway of 18 - 24 months, sufficient to get the Injeq 2.0 on the market.”
Dr. Timo Hänninen, CEO of Injeq Oyj